Geosmin reduction by algaecide application to drinking water: field scale efficacy and mechanistic insights
Heliyon 7 (2021) e07706. Republished with permission. Download PDF.
Authors
David Hammond a,*, Anthony Murri b, Sergey Mastitsky c, Ziming Yang b, Roy Foster d, Linda Schweitzer e
a Earth Science Laboratories, 903 N 47th Street, Rogers, AR 72712, USA
b Department of Chemistry, Oakland University, Rochester, MI 48309, USA
c Next Game Solutions, Sepapaja 6, Tallinn, 15551, Estonia
d City of Tulsa, 3600 Mohawk Blvd, Tulsa, OK 74115, USA
e Southern University Agricultural Research and Extension Center, Baton Rouge, LA 70813, USA
Article Info
Keywords: Geosmin • Taste and odor • Pretreatment • Cyanobacteria • Drinking water • Argosmin
Abstract
Ten years of field data from an Oklahoma drinking water utility were analyzed for the effects of an acid-stabilized, ionic copper algaecide/bactericide called EarthTec on geosmin concentrations in the water traveling by pipeline from the source lake to a water treatment plant. The data show that geosmin already present in the raw water is reduced more during periods of applying algaecide than when not. Median reduction in geosmin concentration from pipe intake to pipe outfall by natural degradation without addition of algaecide was 5.6 ng/L removed (56.7% reduction) and improved to 126 ng/L removed (83% reduction) during periods the algaecide was being dosed at 1 μL/L, equivalent to 0.06 mg/L as copper. A laboratory study to replicate the phenomenon at bench-scale showed that either the algaecide itself or its copper-free acidic carrier can be used to depress pH and drive a reaction converting geosmin to an odorless dehydration product, argosmin. Algaecides intuitively reduce the organisms that produce geosmin, but this study shows that geosmin already present in the water is also being reduced through chemical conversion to the odorless argosmin, representing a novel means of geosmin removal in drinking water.
1. Introduction
Dozens of different chemical compounds have been identified as the source of taste-and-odor problems in drinking water (Khiari, 2004), with the most widespread and persistent of those being secondary metabolites of blue-green algae (cyanobacteria), fungi, and bacteria, especially geosmin (trans-1,10-dimethyl-trans-9-decalol) and 2‑methylisoborneol (MIB) (Izaguirre et al., 1982; Tabachek and Yurkowski, 1976; Watson et al., 2007). Many fungi and soil- and water-borne actinomycetes have also been identified as producers of earthy/musty taste and odor compounds such as geosmin, MIB, and pyrazines, and can potentially grow in intake pipes (Zaitlin and Watson, 2006; Gerber and Lechevalier, 1965; Hill et al., 1995; Juttner and Watson, 2007).
Geosmin and MIB are costly to remove from water, yet their removal is essential because humans find them objectionable at concentrations as low as 10 ng/L (ppt) depending on a variety of factors such as natural sensitivity, previous experience, and background water matrix (Henatsch and Juttner, 1986; Krasner et al., 1983; Pahila and Yap, 2013; Persson, 1980; Rashash et al., 1997). Techniques employed for removal include adsorption to powdered or granular activated carbon, supplementation of activated carbon with potassium permanganate, ozonation (Korth, 1992; Srinivasan and Sorial, 2011; Suffet et al., 1999; Burlingame et al., 1986), and simple dilution with cleaner source waters. Many methods become prohibitively costly when the starting odor concentrations are high. Operational costs of drinking water treatment during a taste-and-odor (T&O) event can be 2.9 times higher than normal, especially when relying on powdered activated carbon (Kishida et al., 2013). Furthermore, given that such low concentrations are still unpalatable, even proven and efficient water treatment processes may not be sufficient to eliminate taste-and-odor (T&O) complaints.
Chlorine-based biocides are commonly used to control the organisms that create T&O compounds (Piriou et al., 2001), yet some of the problematic species are known to be resistant to monochloramine at concentrations commonly used in water disinfection and distribution (Jensen et al., 1994). Use of ultraviolet (UV) photolysis in conjunction with advanced oxidation processes (AOPs) such as hydrogen peroxide or chlorine are used in water disinfection and may help to degrade geosmin and MIB (Ma et al., 2018; Stefan, 2017; Wang et al., 2015) but are not widely used for this purpose. There continues to be an acute need for non-chlorinated agents that reduce the algae and bacteria that cause T&O issues in drinking water and that contribute to disinfection byproducts produced by their reaction with disinfectants.
Chemical conversion represents one avenue for removal of geosmin. Soil microbiologists originally isolated and identified geosmin from actinomycetes and used hydrochloric acid to demonstrate, at bench-scale, that the odor of geosmin can be reduced by conversion to its dehydration product, argosmin (naphthalene,1,2,3,4,4a,5,6,7‑tetrahydro‑4a,8-dimethyl) (Gerber and Lechevalier, 1965) (see Figure 1). Argosmin is odorless at relevant concentrations, so conversion of geosmin to argosmin in drinking water is advantageous from the standpoint of reducing taste and odor.
Citric acid and acetic acid have been used experimentally to acidify distilled water samples and the results demonstrated that the loss of geosmin was correlated with some combination of acid concentration, acid dissociation constant, and final pH (Pahila and Yap, 2013). Final pH was not the sole predictor of performance because addition of 4% acetic acid resulted in the same final pH as 0.1% citric acid (pH about 2), whereas the 4% acetic acid caused on average 96.7% loss of geosmin compared to about 53% loss with 0.1% citric acid. It appeared that citric acid was more efficient in dehydrating geosmin than acetic acid when applied at comparable doses (1% citric acid reduced geosmin by 96.7%) and the authors point out that whereas acetic acid has only one hydrogen atom available per molecule, citric acid has three. However, the study by Pahila and Yap (2013) did not report on the influence of contact time, so the effect of pH over time merits study.
Park (Park, 2013) studied geosmin degradation following application of various acids, including EarthTec, and showed that acidification results in conversion of geosmin to argosmin at reduced pH. Park (2013) reported that water dosed with 0.05 mL/L EarthTec algaecide (i.e., equivalent to 3 mg/L copper) resulted in a 25% loss of geosmin after 48 h, 65% loss after 3 days, and 74% removal after 4 days.
Biodegradation represents another factor and potential tool in management and removal of geosmin. Bacteria living in biofilms have been shown to play a key role in both production (Juttner and Watson, 2007; Zhou et al., 2017) and destruction (Zhou et al., 2011) of geosmin and there is compelling evidence that removal by biodegradation is much faster (half-life of <24 h) and more complete than by volatilization (half-life of 18–35 days) (Li et al., 2012). Sampling and research performed at a full-scale water treatment plant in Japan concluded that strains of Methylobacterium and Oxalobacteraceae living in biofilms within the biological treatment unit were using geosmin as their carbon source for growth and therein reducing geosmin concentrations by an average of 90% during months with favorable temperatures (Xue et al., 2012). The presence of additional carbon sources such as MIB increased the rate of geosmin degradation when compared to samples containing only geosmin and correlated with increased bacterial biomass and activity of adenosine triphosphate (ATP).
1.1. Empirical evidence for EarthTec
Algaecides can be used to reduce the organisms that produce geosmin but there is empirical evidence from some water utilities that geosmin already present in the water may also be reduced by use of algaecide. In scenarios where an acidic algaecide product is applied to waters affected by high counts of cyanobacteria, there could be some conversion of geosmin to argosmin by dehydration due to the acidification of aqueous medium at the site of algaecide application.
In a drinking water treatment plant in the San Francisco Bay region of California where the intake pipe measures less than 1,000 ft, use of the acidic (pH 0.3) copper-based algaecide product EarthTec outside the intake pipe resulted in the utility’s first season in more than 15 years that there were no customer complaints about MIB- and geosmin-related taste and odor, yet other operational practices were unchanged from previous years (personal communication, citation forthcoming).
The focus of this study is the use of EarthTec outside a drinking water intake pipe in Lake Spavinaw, near Tulsa, OK, USA (see Figure 2) for the purpose of geosmin control. Geosmin events in Lake Spavinaw tend to occur in the winter months and dosing of algaecide corresponds to those months, when geosmin levels fluctuate widely due to variations in water quality, nutrient influx, and temperature. Use of the algaecide product has been correlated with successfully reducing geosmin during episodes of cyanobacterial bloom dating back more than 10 years, but the mechanisms of removal have proven difficult to study in the field and had remained unconfirmed. Algaecides cause cyanobacterial cell counts to decline so it is logical that less geosmin would be produced. But the geosmin that had already been produced before algaecide treatment also declined following treatment with EarthTec, with geosmin concentrations decreasing as much as 98% during the approximately 35 h required to traverse the 52 miles from Lake Spavinaw to the terminal reservoir, Lake Yahola, that is adjacent to the drinking water treatment plant (Figure 2).
The recommended EarthTec dosage for control of cyanobacteria is 1-2 parts per million by volume (1–2 μL/L), which is equivalent to 0.06–0.12 mg/L as copper in the treated water. The final pH of any surface water is unaffected by this low dose. However, at the site of algaecide application and before product diffuses and disperses uniformly throughout the water column, the chemical plume forms a temporary concentration gradient and, consequently, a localized reduction in pH that, in theory, could convert geosmin to argosmin.
This study tests the hypothesis that acidic dehydration of geosmin to argosmin is responsible for the observed declines in geosmin in algaecide-treated water. Field data collected by City of Tulsa staff during full-scale operation from 2008 to 2017, with average flows of 60-80 million gallons per day, is presented and results during periods when dosing algaecide are compared to periods when not dosing. At bench-scale it examines the relationship between algaecide dose, contact time, and pH change as a function of the EarthTec (copper-containing) product and its copper-free proprietary carrier in concentrated form. An explanation is proposed for its contribution to observed losses of geosmin in waters treated with the acidic algaecide product.
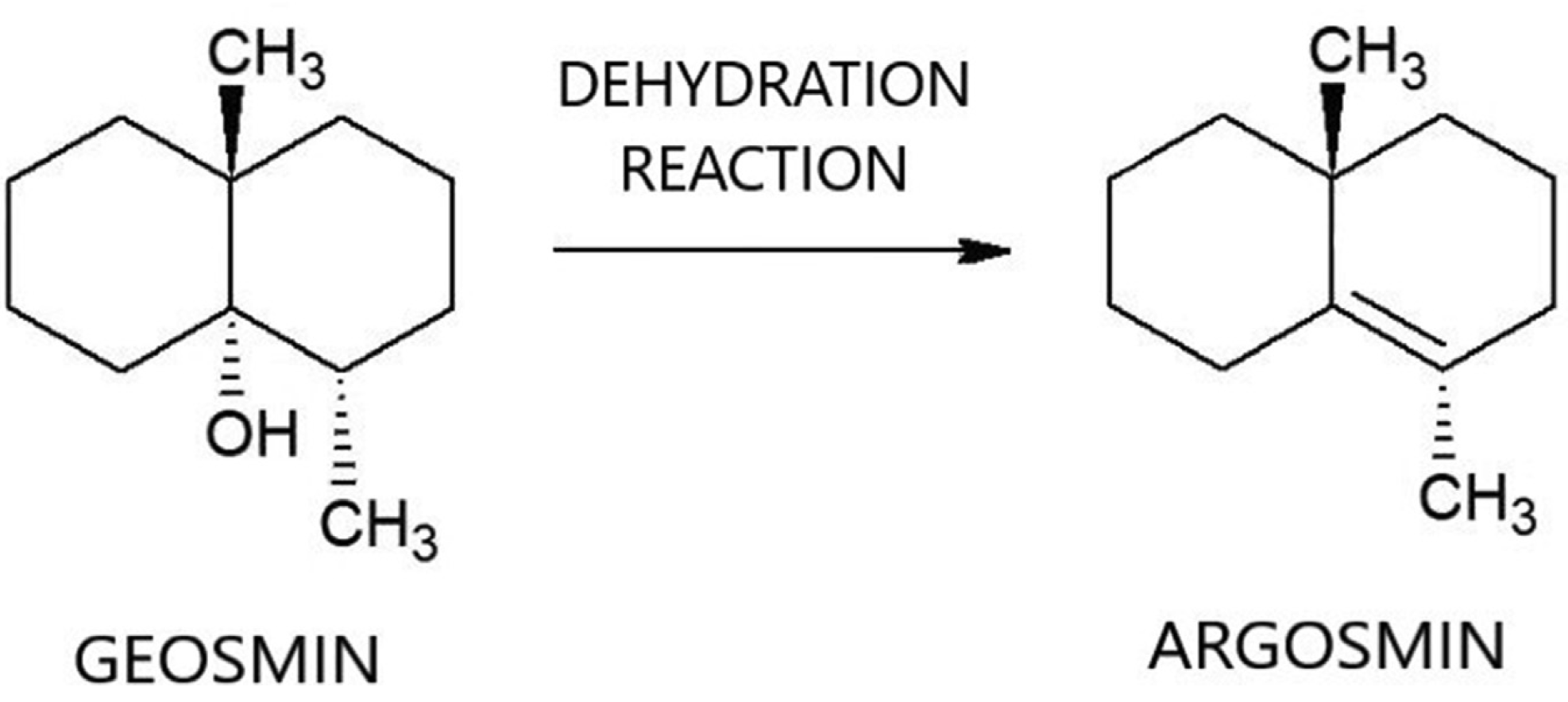
Figure 1. Mechanism for conversion of geosmin to argosmin via dehydration reaction.
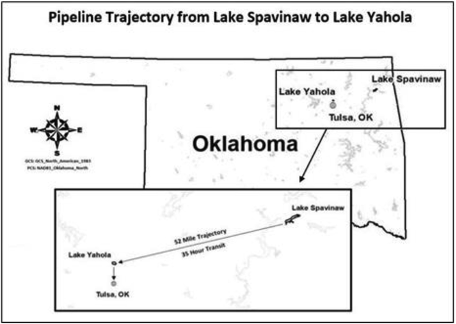
Figure 2. Raw water’s trajectory and transit time from Lake Spavinaw to Lake Yahola reservoir, where the water then enters the Mohawk facility for treatment and subsequent distribution to consumers in Tulsa, Okla.
2. Materials and methods
EarthTec is a liquid, acid-stabilized, ionic copper algaecide containing 5% copper by weight and with a pH of 0.3. The proprietary formulation is derived from 19.8% copper sulfate pentahydrate, small amounts of ammonium sulfate, sulfuric acid, and the remainder made up of water. The specific gravity is 1.188 and the manufacturer’s recommended dose of 1 μL/L as product equates to 0.06 mg/L as elemental copper. The maximum dose permitted by the EPA-accepted label is 16.7 μL/L, equivalent to 1.0 mg/L as copper (EPA Registration #64962‑1). The proprietary base formulation (carrier) of EarthTec, prior to addition of copper sulfate pentahydrate, is known as ET‑3000 and has a pH estimated to be ca. -1.5. Product can be applied directly to open waters, for example at the surface or delivered to a desired depth, or can be injected into the flowing water of an intake structure or pipeline leading to a water treatment facility so that pre-treatment occurs in the pipe. Raw water from Spavinaw varies in quality but alkalinity was typically 120 mg/L and Total Organic Carbon ranged from 2 to 5 mg/L.
2.1. Analysis of field samples from Lake Spavinaw and pipeline outfall
Water samples were taken from the Spavinaw Dam immediately preceding the pipeline’s intake and at the pipe’s outfall into Lake Yahola, a storage reservoir adjacent to the water treatment plant 52-miles away in Tulsa, Okla. The water samples from the field were collected in plastic water bottles and refrigerated until geosmin concentrations were determined within 72 h of collection by Eurofins Eaton Analytical, which performed Solid-Phase Microextraction (SPME) as described in Standard Method 6040D, 21st Edition (APHA 2005).
2.2. Statistical analysis of the historical field data
As the geosmin concentrations in raw source water and at pipeline outfall had highly right-skewed distributions (not shown here), the median was used as a measure of central tendency in data (i.e., as opposed to the arithmetic mean, which is more applicable for symmetric distributions). The bootstrap technique (Efron and Tibshirani, 1994) was used to estimate the median concentrations of geosmin in raw source water and at pipeline outfall when dosing algaecide and when not dosing, as well as the differences between these medians (both in absolute terms and as percent change). For each of these quantities their 95% confidence intervals were estimated using the 0.025th and 0.975th percentiles of the respective bootstrap distributions. Non-overlapping confidence intervals were then considered indicative of a significant difference between the quantities of interest.
Standard bootstrap methodology assumes that the data are independent and identically distributed (Efron and Tibshirani, 1994). However, this is not the case with time series data, which typically demonstrate a considerable degree of autocorrelation. There are several modifications of the standard bootstrap technique that help to preserve the autocorrelation inherent to time series data. In one such modification, a time series is randomly split into non-overlapping blocks of varying length and the bootstrap replicates are then generated by resampling from within each of these blocks (Lahiri, 2003). In the present analysis, a similar approach was used, but instead of random splitting, relied on blocks of geosmin concentrations recorded during the consecutive periods of dosing and not dosing (in total, there were 33 such blocks of varying length for the source water time series and 31 blocks for the pipe outfall times series). By this method 20,000 bootstrap replicates were used to estimate each of the aforementioned quantities.
Raw data contained a large number of geosmin concentrations that were below the detection limit of 5 ng/L (18.5% of all observations in the source water time series, and 46.8% of all observations in the outfall time series). Instead of treating such observations as missing, they were imputed at each bootstrap iteration by randomly sampling from a uniform distribution with a minimum value of 0.01 and a maximum of 4.99. All analyses were conducted using the R statistical computing environment v3.6.2 (R Core Team, 2019). The dataset and a reproducible R script can be obtained upon request.
2.3. Acidification testing of product in different waters
The effect of the algaecide and its carrier on pH of three different water types were evaluated: distilled/deionized (DI); tap water from Oakland County, Mich.; and surface water samples from Tulsa. Tulsa samples were collected during a mild geosmin event (7–12 ng/L) in February 2019 and stored in a refrigerator until used. Experiments with the Tulsa water were conducted within one month of collection. Six dosing levels were evaluated including control (no product added). The pH after dosing of algaecide or carrier and mixing thoroughly was measured by a pH meter (Eutech PCSTestr 35).
2.4. Geosmin dehydration by acidification
Geosmin dehydration by acidification was conducted with food grade citric acid (Milliard Brands, Lakewood, N.J. 08701) to a pH of 1.5 after an analytical standard of /- geosmin (Sigma-Aldrich, St. Louis, Mo.) had been spiked into DI water (MilliQ distilled and deionized) at 1,000-parts per trillion (ppt).
For the dehydration by acidification with algaecide and carrier, the analytical standard of /- geosmin (sigma Aldrich, location) was spiked at various concentrations between 100 ppt and 1000 ppt into DI water, water obtained from the Lake Spavinaw source for City of Tulsa, and tap water from Oakland University, Rochester, Mich., where the bench-scale study was conducted. The water samples were then treated with selected doses of the algaecide product or its carrier, mixed thoroughly once but then left unmixed until being tested for pH and conversion to argosmin. Geosmin was quantified before and after algaecide treatment on split samples according to modified Standard Method 6040D (APHA, AWWA, WEF 2012, 22nd edition): headspace solid phase microextraction (SPME) with GC/MS. The following instrumentation system, GC column and SPME fiber were used: Agilent 7890A-GC/5975C-MS with triple-axis detector, Agilent DB-5ms 30m x 0.25mmID, 0.25um film GC column, and DVB/CAR/PDMS fiber. The method uses selected ion monitoring to quantify geosmin. The quantitation ion was 112 for geosmin and the secondary ion m/z was 125. For identification of argosmin, the 149 and secondary ion 164.2 were viewed but argosmin was not quantified because a pure certified reference standard was unavailable. Argosmin has molecular weight of 182 g/mol and boiling point of 230 oC.
3. Results
3.1. Field data on reduction of geosmin in pipeline
The geosmin concentrations measured at the pipe’s intake and outfall over a period of ten winter seasons and one summer season are shown in Figure 3, where the horizontal green line corresponds to the period of EarthTec dosing, applied at a target dose of 1 μL/L, equivalent to 0.06 mg/L as copper.
During the 10 winter seasons between 2008 and 2017, geosmin concentrations in water samples ranged widely from a minimum of non-detect in most summer months to maxima of 1,300 ng/L in December of 2013 and >3,000 ng/L in December 2014. In 2016 and 2017, utility staff chose not to dose EarthTec because geosmin concentrations in the raw source water were not high enough and/or long-lasting enough to warrant treatment; these 10 years of data comprise more than 600 individual measurements and provide an opportunity to compare geosmin reduction when dosing vs not dosing. The median geosmin concentrations at the Spavinaw intake and the pipeline outfall, along with the median rates of removal, are summarized in Table 1. The median percent removal of geosmin during algaecide dosing was significantly greater than that during no algaecide dosing, as evidenced by the non-overlapping confidence intervals of the two quantities (Figure 4).
Median and average values are not the same, so Table 2 presents the averages of geosmin concentrations and removals, respectively, for samples taken when algaecide was being used versus not being used in surface water treatment.
3.2. Bench-scale data and results
3.2.1. Acidification testing of product in different waters
The geosmin concentrations measured at the pipe’s intake and outfall over a period of ten winter seasons and one summer season are shown in Figure 3, where the effect of the EarthTec algaecide product on the pH of three types of water is shown in Figure 5. Results are reported in v/v of product-to-water as well as dose rate in ppm copper. A copper-free acidic carrier called ET-3000 is used to formulate EarthTec and was also tested for its capacity to acidify DI water, tap water, and Tulsa surface water, and the pH was recorded with a pH meter (Figure 5).
3.2.2. Geosmin degradation by citric and hydrochloric acids
A sample of 20 ppb geosmin in DI water was treated with 1% citric acid by volume, yielding a pH of 1.5. The sample was initially shaken in a vial and then left untouched. After 48 h at room temperature, 31% of the geosminremained, representing a 69% conversion. Analysis by GC-MS revealed two argosmin isomer peaks (i.e., argosmin isomers B and C, according to criteria in Korth (1992) whose areas appeared to account for the 69% loss of geosmin). Longer incubation did not result in complete conversion of geosmin to argosmin. A sample of 1,000 ppt geosmin in DI water was treated with 1% citric acid by volume and after 24 h the geosmin measured had been reduced by 60% versus the untreated control, and the argosmin was 48% of the area of geosmin argosmin on the same sample. Although argosmin and geosmin may have different response factors, the peak areas are consistent with geosmin being converted to argosmin.
Acidification of geosmin with HCl took longer to convert geosmin to argosmin but ended up yielding similar percentages of conversion as citric acid. Loss of geosmin was quantified by measuring geosmin concentration directly, independent of argosmin formation, and appearance of the argosmin peak is interpreted as confirmation that loss of geosmin resulted in the formation of argosmin along with the SIM scan that was set to monitor for both geosmin and argosmin.The mass spectra of the Supelco geosmin standard and for argosmin are presented in Figure 6.
3.2.3. Geosmin degradation by EarthTec® and its copper-free acidic carrier
The Tulsa untreated water used for bench-scale experimentation was analyzed for geosmin and the initial concentration found was 12 ng/L (ppt). Samples of raw Tulsa water that were spiked with 100 ppt geosmin and treated with 4.16 μL/L algaecide (0.25 mg/L copper) showed no effect on sample pH and there was no conversion to argosmin.
Samples of Tulsa water collected in 2019 (pH 7.1) were spiked with 1,000 ppt geosmin and treated with EarthTec at 418 uL/L or with 20 uL/L carrier to reduce pH to 5.3 and 4 respectively after incubation for 48 h. The data are summarized in Figure 7, which includes for comparison the DI sample treated with citric acid to pH 1.5 and one data point from Park (2013), who used water collected at the same Tulsa source. The Tulsa water acidified with carrier to a pH of 4, after 48 h incubation had a geosmin reduction of 56%. At 418 uL/L EarthTec (25 mg/L copper), pH was 5.3 and there was 52% reduction in geosmin. In the replicates treated with carrier, the argosmin peaks doubled in size as compared to the control.
Carrier added at 100 μL/L to different waters that had been spiked with 250 ppt geosmin also resulted in reduction of geosmin that increased with contact time (Figure 8). By 3 h after carrier addition to Tulsa water the geosmin had been reduced by 48.8% and by 29.4% from D.I. water, with modest additional removals occurring up to 48 h. In all samples, losses in geosmin corresponded to increases in argosmin.
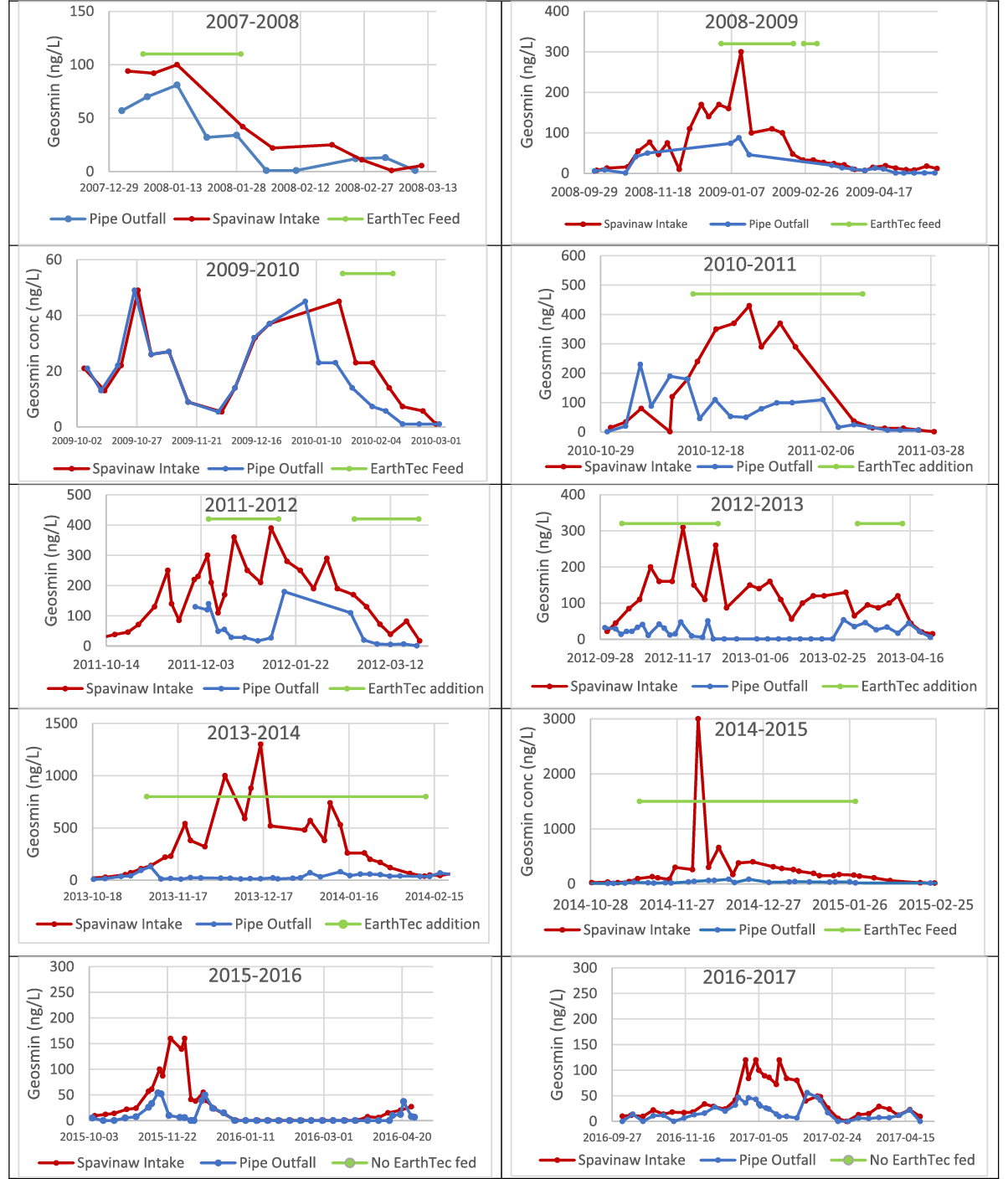
Figure 3. Geosmin concentration (ng/L) over a 10-year period, as measured in the raw water entering the utility’s pipeline (red lines) vs at the pipe’s outfall (blue lines). Horizontal bars indicate the time periods when EarthTec algaecide was being dosed at 1 ppm and each data point is an individual measurement. The data illustrate that some naturally occurring degradation of geosmin sometimes occurs when no algaecide is being fed, but statistical analysis reveals that the removal is significantly greater during algaecide addition.
4. Discussion
4.1. Field data
Statistical analysis confirmed that algaecide applied to Tulsa’s raw water at full-scale reduces the concentration of geosmin already present in the water, yet curiously, comparable levels of reduction could be reproduced at lab scale only by using orders of magnitude higher doses than those used in field operations. The dose of EarthTec needed to reduce the pH of natural waters also far exceeds the doses used by utilities. Still, at the site where algaecide is applied, product will be concentrated before the chemical plume is uniformly dispersed, the pH will be reduced locally, and geosmin within that plume may be converted to argosmin.
The ability of the product to cause the conversion of geosmin to argosmin is a function of dose, pH, and contact time. Where acidic algaecide can be applied to an intake pipe, there may be sufficient concentration and contact time to create a localized pH change and reaction with geosmin.
| Table 1. Median geosmin concentrations (ng/L) and their 95% confidence limits (in parentheses) in raw source water vs at pipeline outfall when dosing algaecide and when not dosing. | ||||
| Median geosmin concentration (ng/L) at: | Intake | Outfall | Median removal (%) | Median removal (ng/L) |
| When no algaecide is fed | 10.0 (9.3, 11.0) | 4.4 (3.9, 4.8) | 56.7 (50.7, 63.5) | 5.6 (4.8, 7.1) |
| When algaecide is fed at 1 μL/L | 150.0 (125.0, 160.0) | 25.5 (22.0, 31.5) | 83.0 (77.7, 86.3) | 126.0 (99.0, 135.5) |
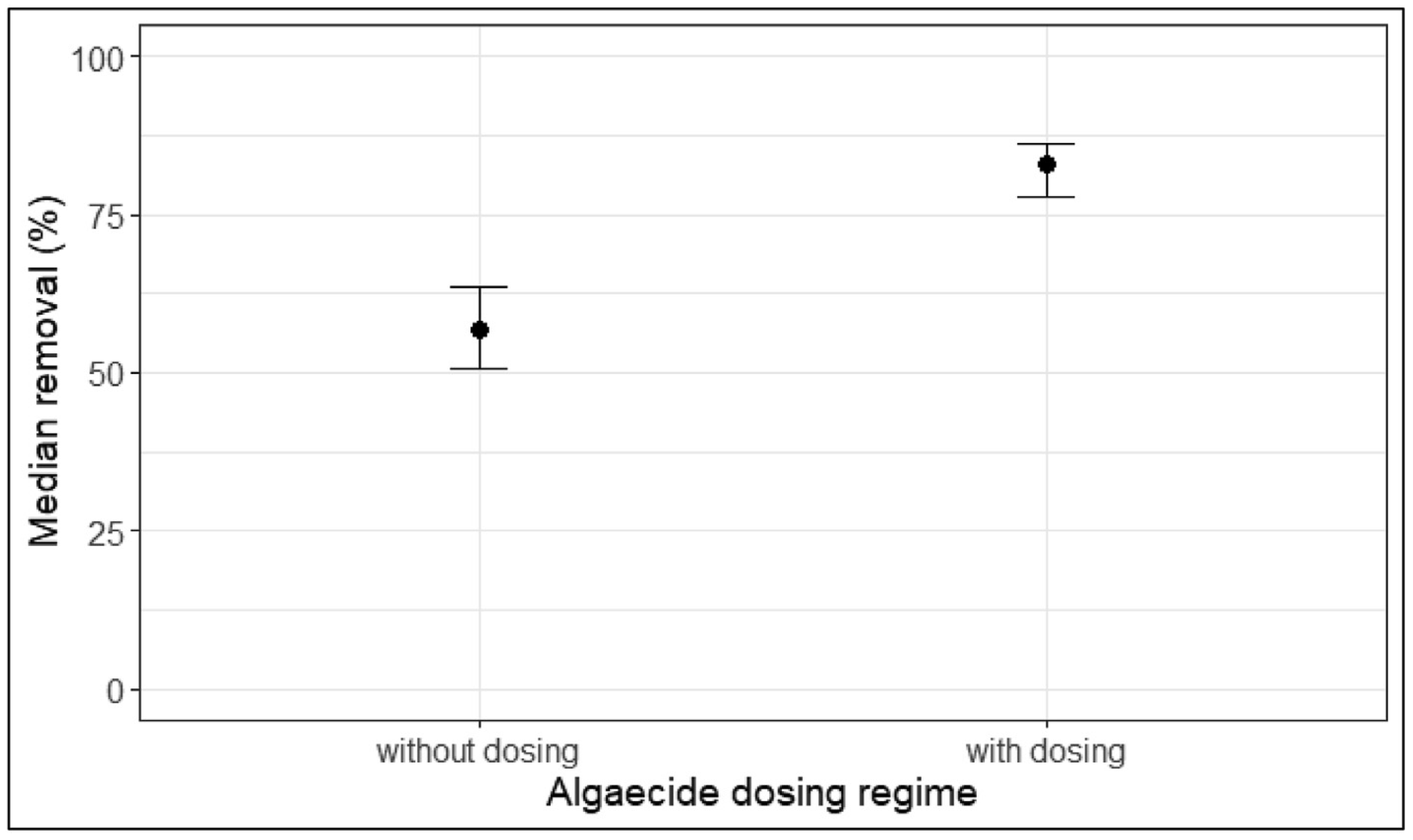
Figure 4. Graph of the estimated 95% confidence intervals using the 0.025th and 0.975th percentiles of the respective bootstrap distributions, wherein the non-overlapping confidence intervals indicate a statistically significant difference between the removal rates observed while dosing algaecide vs while not dosing.
| Table 2. Average geosmin concentrations (ng/L) in raw source water vs at pipeline outfall when dosing algaecide and when not dosing. | ||||
| Average geosmin concentration (ng/L) at: | Intake | Outfall | Average removal (%) | Average removal (ng/L) |
| When no algaecide is fed | 29 (n-516) | 11 (n ¼ 118) | 61.6 | 17.0 |
| When algaecide is fed at 1 μL/L | 241 (n ¼ 103) | 32 (n ¼ 452) | 86.7 | 209 |
4.2. Acidic dehydration of geosmin
EarthTec’s carrier is an inorganic acid more acidic than EarthTec itself and could theoretically be used without copper to lower pH. Carrier alone would not suppress algal growth but could be used during taste and odor events to yield chemical transformation of geosmin to odorless derivatives. The doses of carrier employed in the present laboratory studies would be feasible in a utility-scale treatment process from both economic and regulatory perspectives, so doses of carrier up to 100 μL/L constitute a practical alternative and merit further investigation as a tool for chemically reducing geosmin in drinking water
Addition of EarthTec to water caused a dose-dependent decrease in pH, with the effect being strongest in deionized (DI) water (as expected given its lower buffering capacity), and weakest in raw Tulsa water and Michigan tap water (Figure 6). Addition of the more acidic pure carrier used to formulate EarthTec caused a correspondingly stronger decrease in pH, with the effects again being strongest in deionized water (Figure 6). Note that in real-world field applications, changes in the pH of treated water do not occur because the dose of EarthTec applied for algae control is typically only 1–5 μL/L, and even at the maximum EPA-accepted dose of 16.7 μL/L there was no change in the pH of Tulsa raw water or Michigan tap water.
At lab-scale, the pH needed to be 3 or below to get fifty percent or more conversion of geosmin to argosmin with an incubation time of at least three hours. Shorter incubation times suggest that three hours is an apparent threshold in which geosmin begins to significantly convert to argosmin and at pH greater than 3, it takes longer to achieve high conversions of geosmin to argosmin. Figure 8 shows that regardless of the acid used, the conversion of geosmin to argosmin at 48 h incubation is pH-dependent. There was not much difference between the 25 ppm vs 100 ppm as copper dose (52 vs 56% reduction, respectively) in terms of percent reduction in geosmin at the 48-hour sample point. The treatments appear to work on a percent removal basis that is concentration-independent with regards to geosmin, at least in the range of 25 ppt–1,000 ppt. One potential treatment method would be the use of the algaecide’s copper-free carrier to temporarily depress water pH to about 3 for three hours contact time to eliminate approximately half the geosmin concentration.
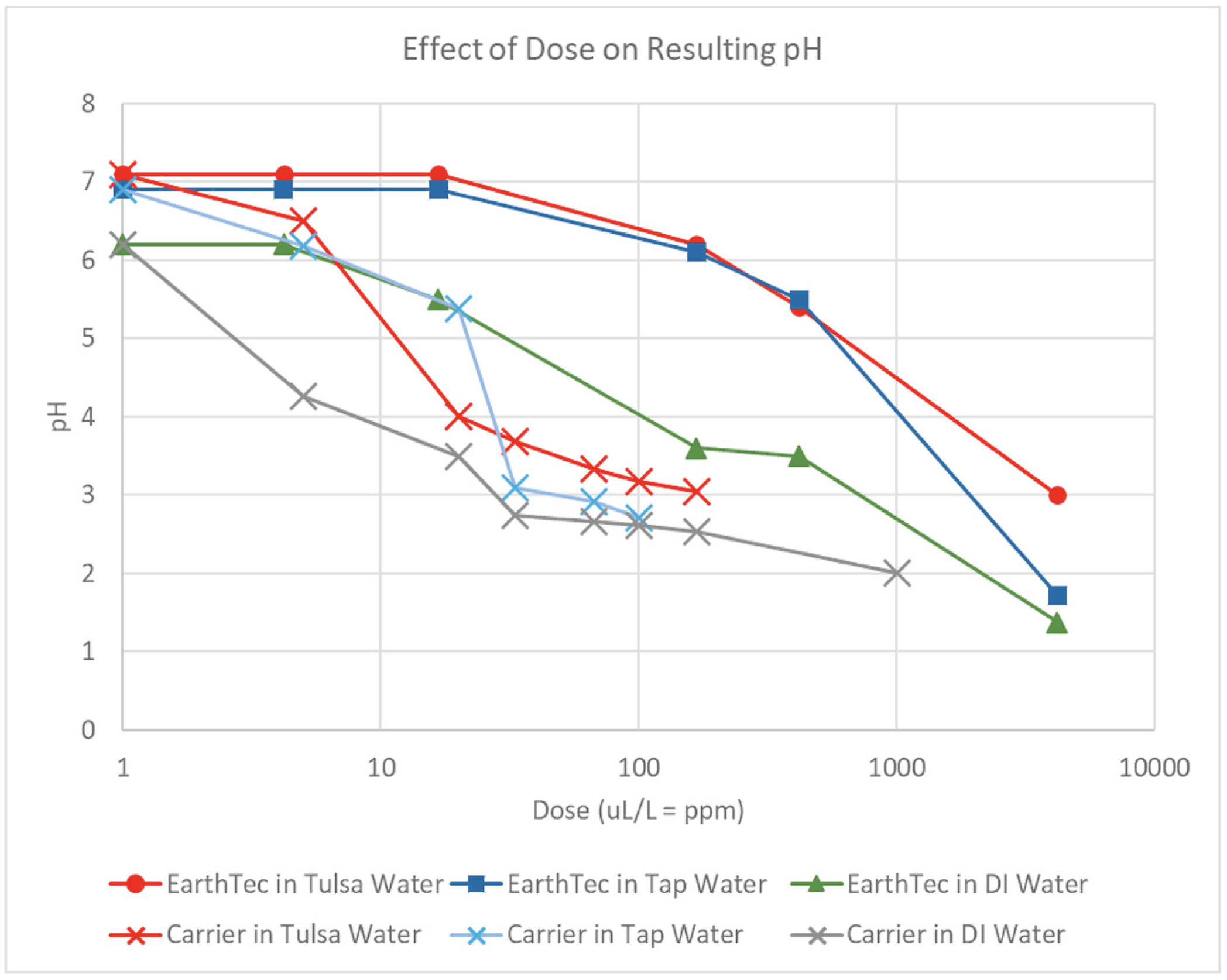
Figure 5. Change in pH as a function of the dose of EarthTec algaecide or its copper-free carrier into different waters.
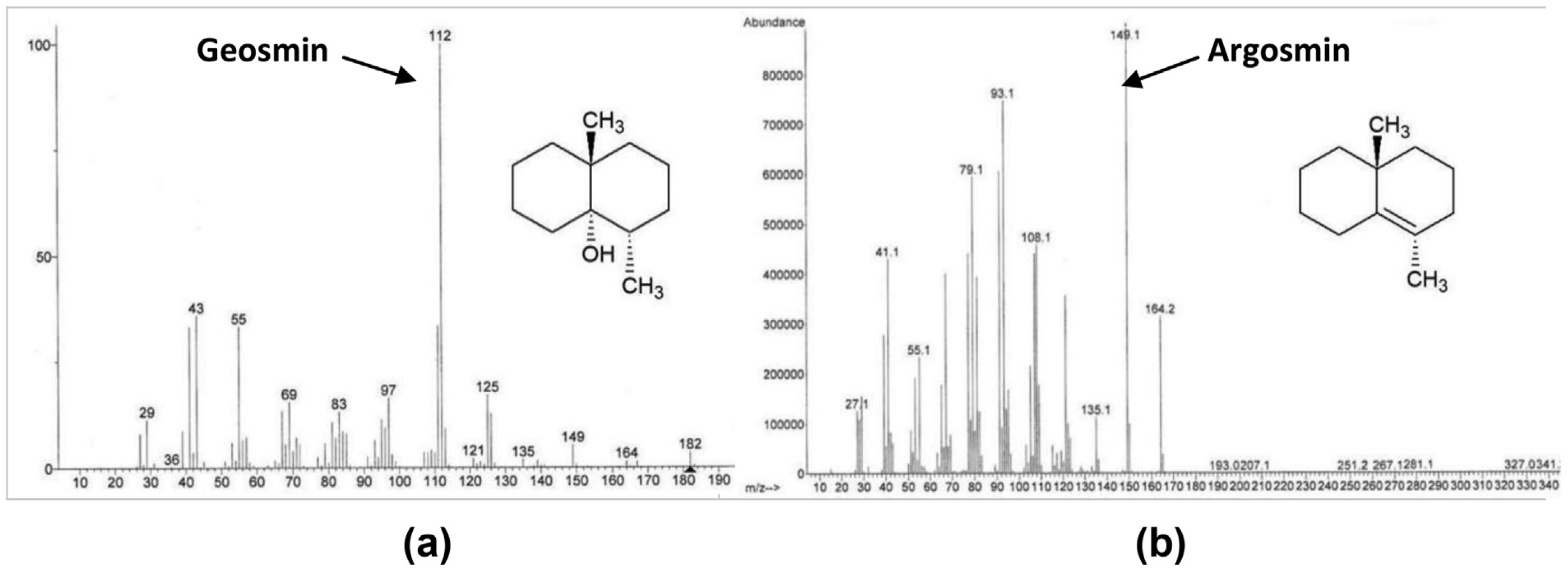
Figure 6. (a) Mass spectrum of geosmin from a Supelco standard analyzed by GC/MS where the m/z of the molecular weight (182) is annotated. (b) Water spiked with the same geosmin (250 ng/L) and then treated with algaecide, showing formation of argosmin.
4.3. Volatilization
Musty, earthy compounds are perceived by humans as very potent, giving the impression that they are very volatile, but geosmin has a low vapor pressure. Air stripping has been tested as a strategy for removal of geosmin and was found ineffective (Lalezary et al., 1984; Zat and Benetti, 2011) unless used in conjunction with traditional powdered activated carbon (Park et al., 2010). Modeling of river mixing by a USEPA EPI SuiteTM program predicts a half-life of 10.51 days from volatilization (https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface). In a closed pipeline with limited headspace, the losses of geosmin during 35 h of transit time can be assumed to be quite low and volatilization is not a meaningful factor in the losses seen at Tulsa.

Figure 7. Geosmin concentration (ng/L) after 48-hour incubation of water samples spiked with 1,000 ng/L geosmin and treated as follows: DI water acidified with 1% citric acid to pH 1.5; Tulsa water treated with Carrier to pH 4; Tulsa water treated with EarthTec to pH 5.3, and data from Park (2013) pH 6.5.
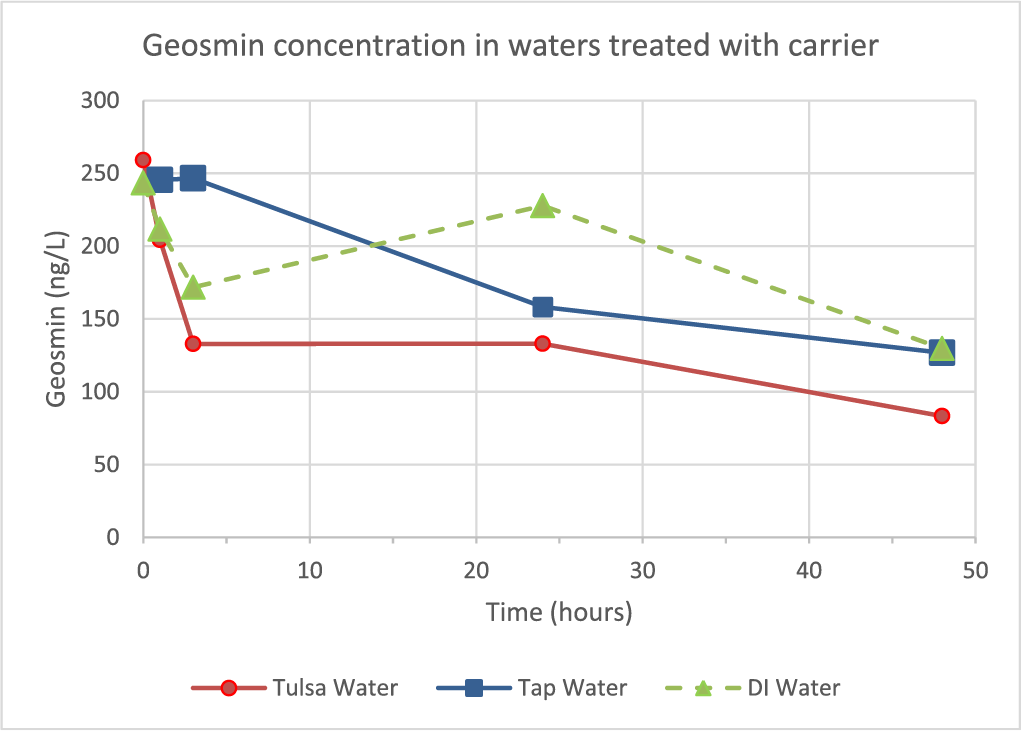
Figure 8. Time dependency of geosmin reduction (from 250 ppt) in different water types dosed with 100 μL/L of ET-3,000, the algaecide’s acidic carrier formulation without copper.
4.4. Biodegradation
Use of the copper-based algaecide EarthTec controls the growth of algae and when fewer algae are present or are no longer producing geosmin, the geosmin concentration in the water can decrease through biodegradation, which can be significant but varies widely depending on the biology present. Conversely, untreated algae will continue to produce geosmin and its production may exceed the degradation rate, resulting in increased total geosmin concentrations, some intracellular and some freely soluble in the water column.
4.5. Effect of acidification on geosmin analysis
The peak areas of the argosmin on the GC/MS chromatogram did not always increase to the same extent as would be expected from the losses of geosmin. The citric acid treated DI water sample had exactly the same increase in argosmin (peak areas of two isomers) as decrease in geosmin (measured by standards addition method), but the concentration of the geosmin was high (1,000 ppt), so the quantification may have been due to better instrument response; note that the standard curve is quadratic at the low end (<25 ppt) of the curve, but linear above 50 ppt. Water samples collected for the analysis of taste-and-odor compounds such as geosmin should not be preserved with acid, as the concentrations will be underestimated. MIB (2‑methylisoborneol) is another earthy/musty metabolite of algae and bacteria that can be dehydrogenated to form a product with a higher odor threshold (Pahila and Yap 2013). Pahila and Yap’s study indicate that MIB is even more sensitive to acidic dehydration than is geosmin.
5. Conclusions
The field data collected at the intake and outfall of the Tulsa pipeline illustrate that use of EarthTec improves removal of geosmin already present in the water, and the losses exceed what can be expected from volatilization alone. Loss of geosmin by acidification and dehydration to argosmin following application of EarthTec remains one likely explanation for the significant total losses observed in the field. Biodegradation of geosmin in the pipe may contribute but if biodegradation were the only explanation there wouldn’t be the statistical improvement measured when dosing EarthTec. Furthermore, Tulsa’s use of copper-containing EarthTec can be expected to diminish biofilms as well as algae, so biodegradation of geosmin by bacteria seems unlikely to be the sole explanation for reduction. Use of the acidic carrier product alone to reduce geosmin merits further investigation.
Scientific studies rooted in the analysis of field data face the inherent handicap that there is no control and no replicates, which reduces the ease of statistical analysis. In the case of City of Tulsa’s source water there is only one pipeline leading from the source at Lake Spavinaw to the water treatment plant, and no second pipeline that could be left untreated, so we can only make comparisons between the intake vs. outfall water and between periods of treating with algaecide and not treating. Nevertheless, the magnitude of the geosmin reductions measured are unexpected, compelling, statistically significant, and have occurred over a period of 10 years. Prior to adopting the use of EarthTec, the utility struggled with high geosmin concentrations at both the pipe’s intake and outfall.
Geosmin can be converted to its dehydration product, argosmin, and the percent conversion is a function of pH and contact time. The algaecide EarthTec is an acidic liquid formulation of ionic copper, and the carrier liquid is more acidic than the final copper-containing product. It takes a lower dose of carrier alone than of copper-formulated product to achieve a given reduction in pH and conversion of geosmin to argosmin. Other acids will convert geosmin to argosmin, and citric acid was used previously, albeit at the very high dose of 10,000 μL/L, to demonstrate pH effect as the mechanism for conversion. It appears that the pH of the water is a reasonable predictor of geosmin loss when contact time is held constant (e.g., 48 h in this study).
This study also demonstrates that water samples collected for the analysis of taste-and-odor compounds should not be preserved with acid, as the original geosmin and MIB concentrations will likely be underestimated.
Despite the many advances achieved in water treatment technology, T&O events continue to create episodes of great urgency, concern, complaints, and cost. More research is needed for a better understanding of the mechanisms responsible for biosynthesis, biodegradation, and chemical destruction of T&O compounds. Algal, bacterial, and cyanobacterial metabolites in the raw water are known sources of T&O compounds, but measured concentrations can also increase within the treatment facility and the distribution system. Alternative treatment methods that reduce reliance on chlorine chemistry stand to reduce the inadvertent creation of odorous chlorinated compounds and halogenated disinfection by-products such as trihalomethanes and haloacetic acids. Ionic copper is a proven antimicrobial agent that is not associated with formation of disinfection by-products and can be effective at rates as low as 5% of the regulatory standard for drinking water (Lead and Copper Rule), making it a valuable tool for consideration in drinking water management.
Declarations
Author contribution statement
- David Hammond: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
- Anthony Murri: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
- Sergey Mastitsky: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
- Ziming Yang: Analyzed and interpreted the data.
- Roy Foster: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
- Linda Schweitzer: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare the following conflict of interests: David Hammond; [David Hammond is an employee of Earth Science Laboratories, manufacturer of the algaecide that was utilized in this study.]
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express their appreciation to Hua Jiang and Jennifer Lindly with the City of Tulsa for their collaboration in collecting and sharing the field data and to Renata Claudi and Fred Singleton for their review of the manuscript and helpful comments.
References
- APHA, 2005. Standard Methods for the Examination of Water and Wastewater, twenty-first ed. American Public Health Association/American Water Works Association/ Water Environment Federation, Washington DC.
- Burlingame, G.A., Dann, R.M., Brock, G.L., 1986. A case study of geosmin in Philadelphia’s water. J. Am. Water Works Assoc. 78, 56–61.
Efron, B., Tibshirani, R.J., 1994. An Introduction to the Bootstrap. Chapman and Hall/ CRC. - EarthTec product label registered with US environmental protection agency. accessible at. https://www3.epa.gov/pesticides/chem_search/ppls/064962-00001-20210203.pdf.
- Gerber, N.N., Lechevalier, H.A., 1965. Geosmin, an earthy-smelling substance isolated from actinomycetes. Appl. Microbiol. 13, 935–938.
- Henatsch, J.J., Juttner, F., 1986. Production and degradation of geosmin in a stratified lake with anaerobic hypolimnion (Schleinsee). FEMS Microbiol. Lett. 35, 135–139.
- Hill, J.L., Hocking, A.D., Whitfield, F.B., 1995. The role of fungi in the production of chloroanisoles in general purpose freight containers. Food Chem. 54, 161–166.
- Izaguirre, G., Hwang, C.J., Krasner, S.W., McGuire, M.J., 1982. Geosmin and 2‑methylisoborneol from cyanobacteria in three water supply systems. Appl. Environ. Microbiol. 43, 708–714.
- Jensen, S.E., Anders, C.L., Goatcher, L.J., Perley, T., Kenefick, S., Hrudey, S.E., 1994. Actinomycetes as a factor in odour problems affecting drinking water from the North Saskatchewan River. Water Res. 28, 1393–1401.
- Juttner, F., Watson, S.B., 2007. Biochemical and ecological control of geosmin and 2‑methylisoborneol in source waters. Appl. Environ. Microbiol. 73, 4395–4406.
- Khiari, D., 2004. AWWA’s taste and odor committee: seeks to understand and solve taste and odor problems in drinking water. J. Am. Water Works Assoc. 96, 32–36.
- Kishida, N., Konno, Y., Nemoto, K., Amitani, T., Maki, A., Fujimoto, N., Akiba, M., 2013. Recent trends in microorganism-related off-flavor problems in drinking water treatment systems in Japan. Water Sci. Technol. Water Supply 13, 1228–1235.
- Korth, W., 1992. Determination of Odour Compounds in Surface Waters.
- Krasner, S.W., Hwang, C.J., McGuire, M.J., 1983. A standard method for quantification of earthy-musty odorants in water, sediments, and algal cultures. Water Sci. Technol. 15, 127–138.
- Lahiri, S.N., 2003. Resampling Methods for Dependent Data, Springer Series in Statistics. Springer-Verlag, New York.
- Lalezary, S., Pirbazari, M., McGuire, M.J., Krasner, S.W., 1984. Air stripping of taste and odor compounds from water. J. Am. Water Works Assoc. 76, 83–87.
- Li, Z., Hobson, P., An, W., Burch, M.D., House, J., Yang, M., 2012. Earthy odor compounds production and loss in three cyanobacterial cultures. Water Res. 46, 5165–5173.
- Ma, L., Wang, C., Li, H., Peng, F., Yang, Z., 2018. Degradation of geosmin and 2‑methylisoborneol in water with UV/chlorine: influencing factors, reactive species, and possible pathways. Chemosphere 211, 1166–1175.
- Pahila, J.G., Yap, E.E.S., 2013. Reduction of off-flavour compounds (geosmin and 2‑methylisoborneol) using different organic acids. Aquacult. Aquar. Conserv. Legis. – Int. J. Bioflux Soc. 6, 510–517. http://www.bioflux.com.ro/docs/2013.511-517.pdf.
- Park, H., 2013. Removal of Geosmin and 2‑methylisoborneol Using Algaecides and Chemicals in Potable Water. Oklahoma State University, Stillwater, OK.
- Park, S.-M., Heo, T.-Y., Park, N.-B., Na, K.-J., Jun, H.-B., Jung, J.-Y., 2010. Application of air stripping to powdered activated carbon adsorption of geosmin and 2‑methylisoborneol. J. Water Supply Res. Technol. – Aqua 59, 492–500.
- Persson, P.-E., 1980. Sensory properties and analysis of two muddy odour compounds, geosmin and 2‑methylisoborneol, in water and fish. Water Res. 14, 1113–1118.
- Piriou, P., Malleret, L., Bruchet, A., Ki,en,e, L., 2001. Trichloroanisole kinetics and musty tastes in drinking water distribution systems. Water Sci. Technol. Water Supply 1, 11–18.
- R Core Team, 2019. A Language and Environment for Statistical Computing [WWW Document]. URL. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing. (Accessed 2 December 2020).
- Rashash, D.M.C., Dietrich, A.M., Hoehn, R.C., 1997. FPA of selected odorous compounds. J. Am. Water Works Assoc. 89, 131–141.
- Srinivasan, R., Sorial, G.A., 2011. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: a critical review. J. Environ. Sci. 23, 1–13.
- Stefan, M. (Ed.), 2017. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications. IWA Publishing.
- Suffet, I.H. (Mel), Khiari, D., Bruchet, A., 1999. The drinking water taste and odor wheel for the millennium: beyond geosmin and 2‑methylisoborneol. Water Sci. Technol. 40, 1–13.
- Tabachek, J.-A.L., Yurkowski, M., 1976. Isolation and identification of blue-green algae producing muddy odor metabolites, geosmin, and 2‑methylisoborneol, in Saline lakes in Manitoba. J. Fish. Res. Board Can. 33, 25–35.
- Wang, D., Bolton, J.R., Andrews, S.A., Hofmann, R., 2015. Uv/chlorine control of drinking water taste and odour at pilot and full-scale. Chemosphere 136, 239–244.
- Watson, S.B., Charlton, M., Rao, Y.R., Howell, T., Ridal, J., Brownlee, B., Marvin, C., Millard, S., 2007. Off flavours in large waterbodies: physics, chemistry and biology in synchrony. Water Sci. Technol. 55, 1–8.
- Xue, Q., Shimizu, K., Sakharkar, M.K., Utsumi, M., Cao, G., Li, M., Zhang, Z., Sugiura, N., 2012. Geosmin degradation by seasonal biofilm from a biological treatment facility. Environ. Sci. Pollut. Res. Int. 19, 700–707.
- Zaitlin, B., Watson, S.B., 2006. Actinomycetes in relation to taste and odor in drinking water: myths, tenets, and truths. Water Res. 40, 1741–1753.
- Zat, M., Benetti, A.D., 2011. Removal of the odoriferous compounds geosmin and 2‑methylisoborneol from drinking water by the processes of cascade aeration, air stripping and nanofiltration (Remoção dos compostos odoríferos geosmina e 2‑metilisoborneol de, aguas de abastecimento através de processos de aeração em cascata, dessorção por ar e nanofiltração). Eng. Sanit,aria Ambient. 16, 353–360.
- Zhou, B., Yuan, R., Shi, C., Yu, L., Gu, J., Zhang, C., 2011. Biodegradation of geosmin in drinking water by novel bacteria isolated from biologically active carbon. J. Environ. Sci. 23, 816–823.
- Zhou, X., Zhang, K., Zhang, T., Li, C., Mao, X., 2017. An ignored and potential source of taste and odor (T&O) issues—biofilms in drinking water distribution system (DWDS). Appl. Microbiol. Biotechnol.
* Corresponding author
E-mail address: dhammond@earthsciencelabs.com, david@biomimico.com (D. Hammond).
https://doi.org/10.1016/j.heliyon.2021.e07706
Received 11 May 2021; Received in revised form 1 July 2021; Accepted 29 July 2021
2405-8440/© 2021 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/bync-nd/4.0/).